Ion Source and Reactive Depositions
The purpose of the ion source is self-explanatory: it creates ions, or atoms that have one or more electrons stripped off. There are two parts to the ion source: the electron source and the anode. Electrons are emitted by the source and get accelerated towards the anode; along their path, they strike atoms/molecules in the "working gas" (which flows into the cone of the anode), knocking out electrons and thus ionizing them. The ions are then accelerated to energies of about 40-70 eV each. The ion souce is directed so most ions hit the substrate. Our particular ion source is a Kaufmann and Robinson EH-2000 with a hollow-cathode electron source and water-cooled anode.
Our ion source is used for two purposes in the chamber. The first is to "ion clean" the substrate before deposition. The ions literally knock water molecules off the surface of the substrate, which helps the coating to bond more tightly with the surface. Also, if there is oxygen in the working gas, it can "burn off" any hydrocarbons that may be left on the substrate.
The second use is for
IAD has another very important mode:
it is used for "
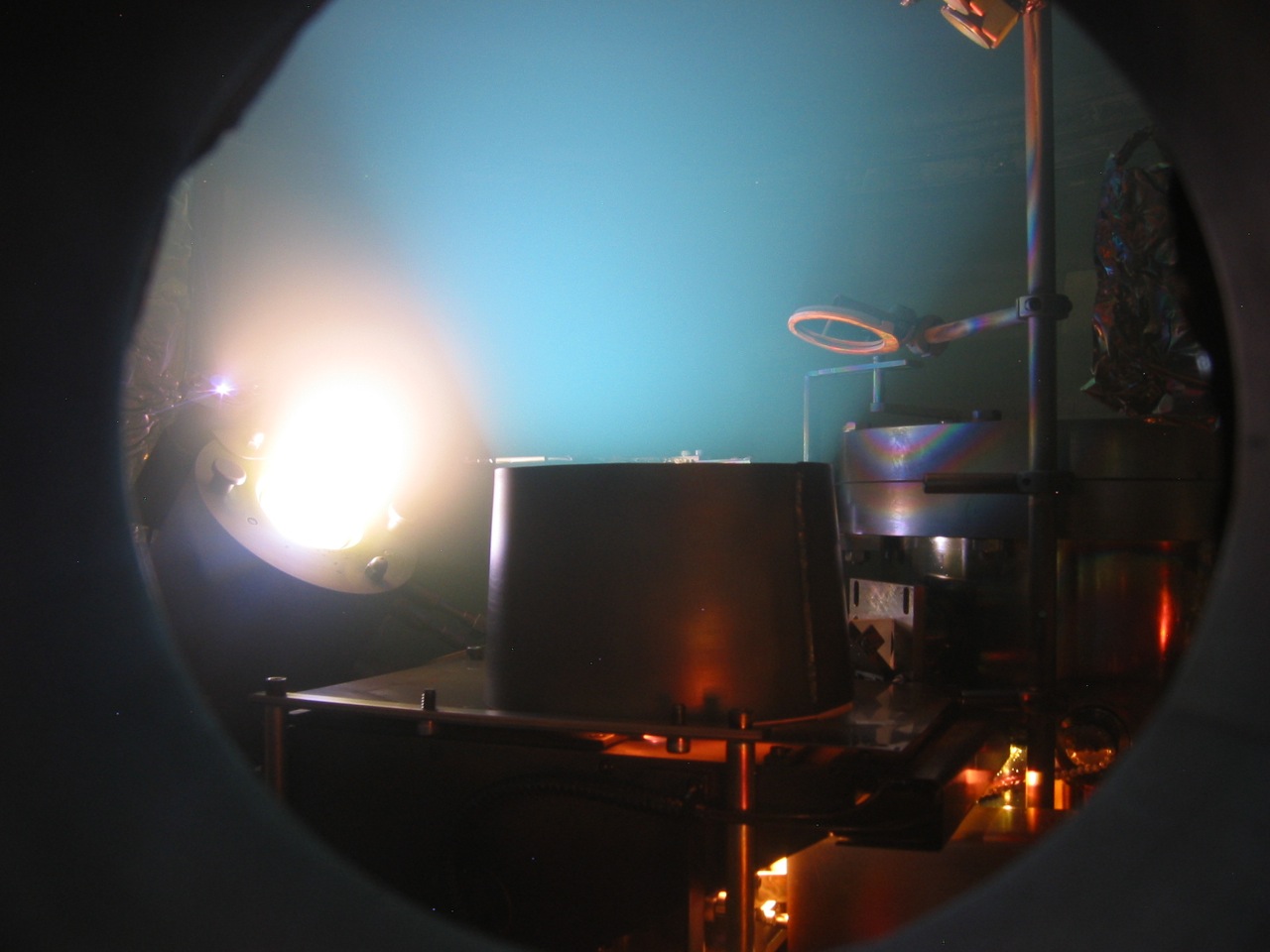
|
(left) View into the chamber from the side viewport during reactive deposition of titanium oxide. The e-gun (under the chimney baffle) deposits titanium metal while the substrate is being bombarded by oxygen ions coming from the ion source (left). Some of the ions also strike the titanium vapor plume coming from the e-gun, causing it to glow light blue. |
The Advanced Coatings Lab has received National Science Foundation support via Award AST-1005506. NSF is not responsible for the content of this website.